Distillation Technique Quiz
1) The literature reports a boiling point for cyclohexanol of 161°. In Boulder, Colorado, the atmospheric pressure is usually about 625 mm Hg. In Boulder, what is the observed boiling point of cyclohexanol?
Show answer
If cyclohexanol boils at 161° at 625 mm, the value that would be obtained at standard temperature/pressure is calculated as follows:
Tcorr = Tobs + 0.00010(760-p)(Tobs + 273)
161 = Tobs+ 0.00010(760-625)(Tobs + 273)
161 = Tobs + 0.0135Tobs + 3.686
Tobs = 155
You can also use the rule of thumb. 760-625 = 135 or 13.5 10 mm pressure increases, so the expected boiling temperature at 760 mm would be 161 - 13.5 x 0.5° or 154°.
2) You have been given an unknown compound by your TA and told only that it is an ester. You determine the boiling point by simple distillation to be 133°. The atmospheric pressure is 625 mm. What is the corrected boiling point of the compound? For fun, look in the table of physical data at the end of the Handbook and determine which ester it might be (it smells like bananas).
Show answer
Tcorr = Tobs + 0.00010(760-p)(Tobs + 273)
Tcorr = 133 + 0.00010(760-625)(133 + 273)
Tcorr = 138.5°
Again, you can also use the rule of thumb. 760-625 = 135 or 13.5 10 mm pressure decreases, so the expected boiling temperature at 760 mm would be 133 + 13.5 x 0.5° or 140°.
The reason we had to convert to the boiling point at 760° is because that is the value reported in the tables of physical data. Look in the tables at the back of the Handbook for a compound that is an ester which boils at about 138-140°. The closest one that is an ester is "acetic acid, 3-methylbutyl ester" (a.k.a. isobutyl acetate) at 142°. It is not unusual for the corrected value of a boiling point taken in our labs to be a little lower than the value reported in the literature.
3) Which of the following compound pairs could be separated by simple distillation?
- acetone and aniline
- butyl acetate and butanol
- cyclohexane and cyclohexanol
- hexanes and toluene
Show answer
First, look up the boiling points of the compounds. They are all in the tables at the back of the Handbook, although butyl acetate is listed as "acetic acid, butyl ester". If the boiling points differ by at least 70°C, they can be separated by simple distillation.
|
bp, °C |
|
bp, °C |
Separable? |
| Acetone |
56 |
Aniline |
184 |
Yes |
| Butyl acetate |
126 |
Butanol |
117 |
No |
| Cyclohexane |
81 |
Cyclohexanol |
161 |
Yes |
| Hexanes |
69 |
Toluene |
111 |
No |
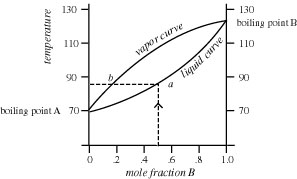
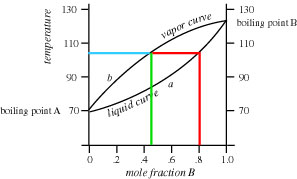
4) Refer to the figure below. If the mole fraction of B in the liquid is 0.8, what is the composition of B in the vapor above the liquid? What will be the reading on the thermometer at this point?
Show answer
At mole fraction B of 0.8, the composition of the vapors is 0.45 mole fraction B and the thermometer reading of the vapors is about 103°C. These numbers are obtained by drawing a vertical line up from the point on the X-axis corresponding to mole fraction 0.8 of B to the intersection with the bottom (liquid) curve (red vertical line). Then, draw a horizontal line from this point to the intersection point with the upper (vapor) curve (red horizontal line). From this point, read down to the X-axis to read "0.45" (green vertical line) and left to the Y-axis to read "103°C"(blue horizontal line).
5) You prepare a fractionating column by filling the condenser in your lab drawer with glass beads. The glassware in the teaching labs is not entirely uniform from one student drawer to the next, and you notice that when your neighbor packs his condenser with glass beads, he is able to fit a lot fewer beads into the condenser than you are. Whose column will be more efficient as a fractionating column, and why?
Show answer
The greater the interior surface area of a fractionating column, the greater the number of theoretical plates and thus the greater the efficiency of the fractionating column. Since your neighbor's column has fewer glass beads, that column would have less interior surface area and it would be less efficient than yours.
6) Why is a fractionating column packed with glass beads more efficient than a Vigreux column of the same length and same diameter?
Show answer
The Vigreux column has less interior surface area than one packed with glass beads and thus is less efficient.
7) While distilling 50 mL of aniline which you prepared in the lab by a procedure involving an aqueous reaction, you record a boiling point (in Colorado) of 92°. The temperature remains at this value throughout most of the distillation. However, the distillate drips into the receiving flask as cloudy drops, and separates in the flask as two layers. Explain.
Show answer
A boiling point of 92°C corresponds to about 98°C literature value. Aniline, however, boils at 184°C. The reason for the lower boiling point is that the aniline, prepared in the lab in an aqueous reaction, is mixed with water and an azeotrope is formed. This azeotrope is a constant boiling mixture of 81% water and 19% aniline. The two boil together at 98°C (STP), but when they condense, they are not miscible and form two layers. The layers are cloudy because there is still some aniline in the water and some water in the aniline. (The azeotropic data for an aniline/water mixture is given in the table at the end of the Handbook section on Azeotropes.)
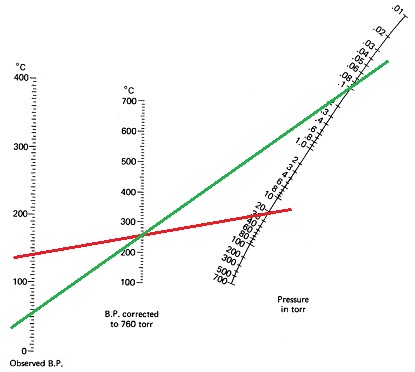
8) Eugenol (clove oil) has a reported boiling point of 255°C at 760 mm Hg. When determining the value of a boiling point at the greatly reduced pressures used in vacuum distillations, you must use a nomography chart rather than the boiling point correction formulas. Use the nomogram given below to determine the boiling point under:
- water aspirator vacuum, about 25 torr
- mechanical pump vacuum, about 0.1 torr
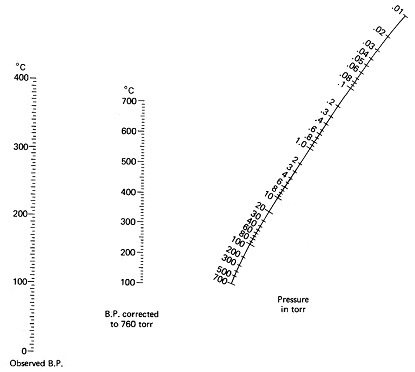
Show answer
- a) At 25 torr, eugenol will boil at about 145°C (red line)
- b) At 0.1 torr, eugenol will boil at about 55°C (green line)
9) Each of the distillation set-ups pictured below has something wrong with it. Figure out what the problem is for each one.
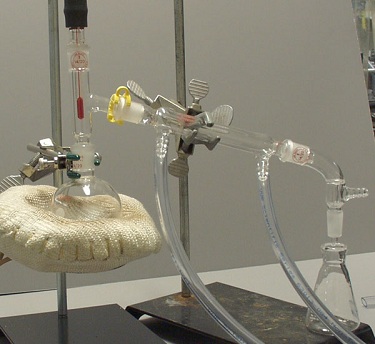
Set-Up 1 |
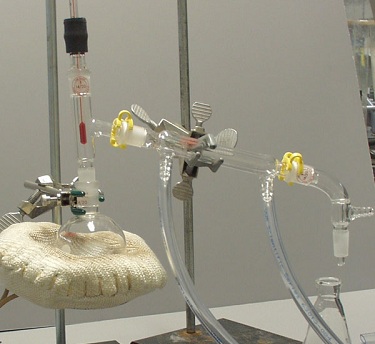
Set-Up 2 |
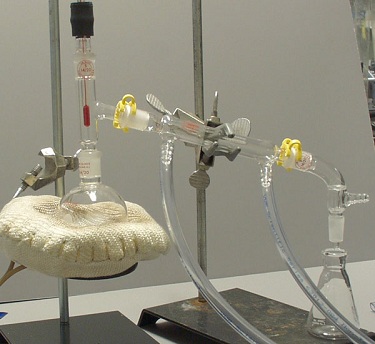
Set-Up 3 |
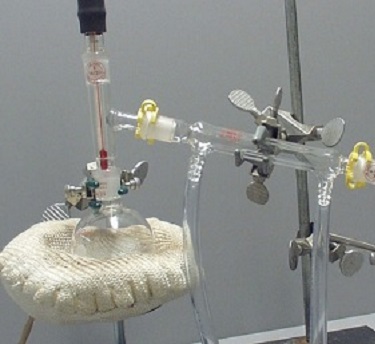
Set-Up 4 |
Show answer
Set-Up 1: There is no Keck clip holding the vacuum adaptor onto the condenser.
Set-Up 2: The vacuum adaptor is not directly over the receiving flask, and the round-bottom flask is not properly connected to the Y-adaptor.
Set-Up 3: The round-bottom flask is not attached to the ring stand with a three-prong clamp.
Set-Up 4: The thermometer is positioned too low.
Back to Distillation








