Problem 14
Formula: C8H14O


C8H14O
Rule 2, omit O, gives C8H14
8 - 14/2 + 1 = 2 degrees of unsaturation.
Look for any 2 of pi bonds or aliphatic rings.
The band at 1718 indicates a carbonyl, probably a ketone. The bands at 3000-2850 indicate C-H alkane stretches. Since the compound is an alkene, one would expect to see C=C stretch at 1680-1640; these weak bands are not seen in this IR (according to Silverstein, "the C=C stretching mode of unconjugated alkenes usually shows moderate to weka absorption at 1667-1640"). Since the compound is an alkene, C-H stretch should appear above 3000 (not seen: the absorption for this single hydrogen must be too weak).
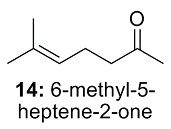
This is the structure. See if you can assign the peaks on your own.

Here, the two E methyl groups have different chemical shifts because they are permanently locked into different positions with respect to the alkene.
